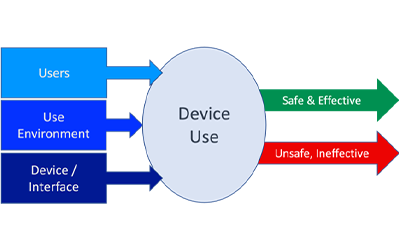
Regulatory reform of Laboratory Developed Tests (LDT) has been a long time in the making, but lawmakers and industry groups alike are debating to what degree of oversight is appropriate. Legislation called the VALID (Verifying Accurate Leading-edge IVCT Development)...