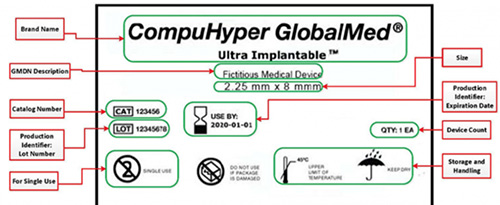
In October 2019, FDA released a guidance suggesting new labeling and performance testing relative to 510(k) premarket notification submissions for coronary, peripheral, and neurovascular guidewires under the product codes DQX and MOF. According to FDA, these new...