MEDIcept Insights
Electronic Submissions For Medical Devices
On July 15, 2020, FDA released a guidance regarding electronic submissions for medical devices as well as their plans for implementing section 745A(b)(3) of the FDA Reauthorization Act of 2017 (FDARA).
FDA Publishes “Review and Update of Device Establishment Inspection Processes and Standards” Guidance
The FDA has published the final version of a guidance document it initially issued in March 2019 entitled “Review and Update of Device Establishment Inspection Processes and Standards.” The document outlines how the agency plans to implement uniform procedures for...
Pre-Sub Process Recommendations
A Pre-Submission (or Q-Submission) is a structured process for developing interactions between medical device manufacturers and FDA about a myriad of topics that ultimately point to future applications for approval or clearance, prior to the IDE, 510(k), PMA, or De...
New and Important Distributor Requirements Under EU MDR
European Union (EU) medical device regulations (EU MDR) have significantly increased the requirements for distributors. If you are currently a distributor of medical devices in the EU, or are considering doing so, it is important that you understand these new...
Coronary, Peripheral, and Neurovascular Guidewires – Performance Tests and Recommended Labeling
In October 2019, FDA released a guidance suggesting new labeling and performance testing relative to 510(k) premarket notification submissions for coronary, peripheral, and neurovascular guidewires under the product codes DQX and MOF. According to FDA, these new...
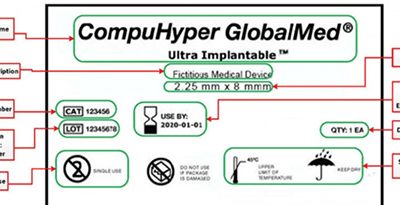
New Unique Device Identifier Deadline Approaches
A Unique Device Identifier (UDI) is a numeric or alphanumeric code that is assigned to a medical device. These codes are entirely unique, designed as a method to easily identify and determine the use of devices sold in the United States and Europe. The purpose of a...